Reata Pharmaceuticals
Reata
Pharmaceuticals (Reata) is a biopharmaceutical company based in Irving, Texas
with over 100 current employees [1]. Their development program has produced
several late-stage therapeutic candidates for the treatment of serious diseases
with significant unmet needs although none of their drugs have reached the
market yet.
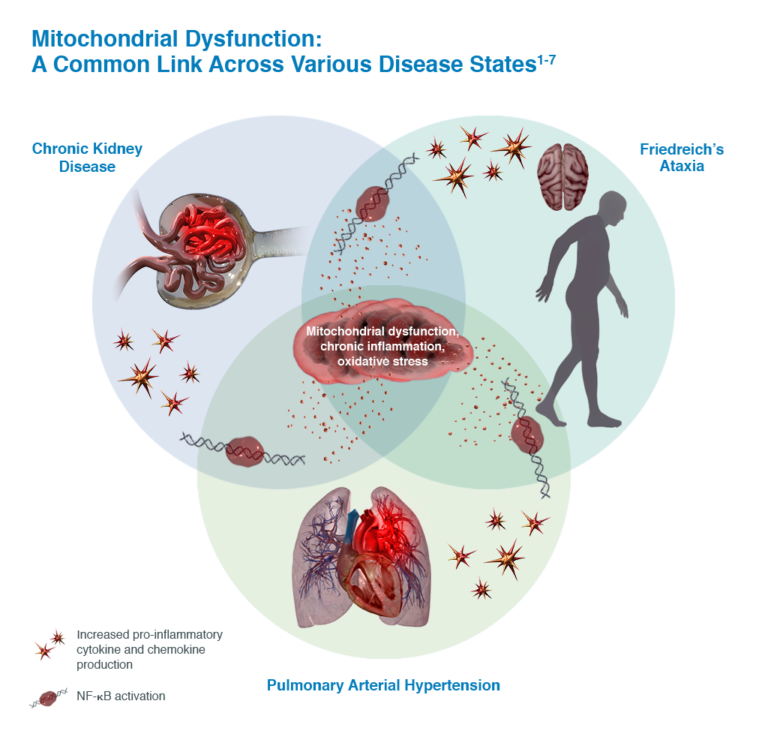
Reata was founded in 2002 with research pioneered at UT Southwestern Medical Center in Dallas and at UT M.D. Anderson Cancer Center in Houston by the following scientists: Dr. Jef Karel DeBrabander, Assistant Professor of Biochemistry at UT Southwestern; Dr. Jonathan M. Graff, Associate Professor in the Center for Developmental Biology and of Molecular Biology at UT Southwestern; Dr. Waldemar Priebe, Professor of Medicinal Chemistry at UT M.D. Anderson; Dr. Thomas Südhof, Professor of Molecular Genetics and Director of the Center for Basic Neuroscience at UT Southwestern; Dr. Philip J. Thomas, Associate Professor of Physiology at UT Southwestern; and Dr. Jerry W. Shay, Professor of Cell Biology at UT Southwestern [2]. Reata's current lead pivotal programs include Alport syndrome, Friedreich's Ataxia, autosomal dominant polycystic kidney disease (ADPKD), and pulmonary arterial hypertension associated with connective tissue diseases (CTD-PAH). These are all chronic diseases characterized by continuous inflammation and mitochondrial dysfunction [3].
Reata's two lead candidates are bardoxolone methyl and omaveloxolone that are both known to activate nuclear factor erythroid 2-related factor 2 (NRF2) [4]. NRF2 is a critical transcription factor in the antioxidative stress response and activates cytoprotective genes related to redox reactions and detoxification to restore cellular homeostasis [5]. During non-stressed situations, NRF2 is bound by the Kelch-like ECH-associated protein 1 (KEAP1), targeting it for degradation by the ubiquitin proteasome pathway [6]. Bardoxolone methyl and omaveloxolone both function to prevent KEAP1-mediated degradation of NRF2, leading to upregulation of NRF2 transcriptional programming; additionally, bardoxolone methyl and omaveloxolone can inhibit NF-kB-mediated inflammation (see figure below) [7].
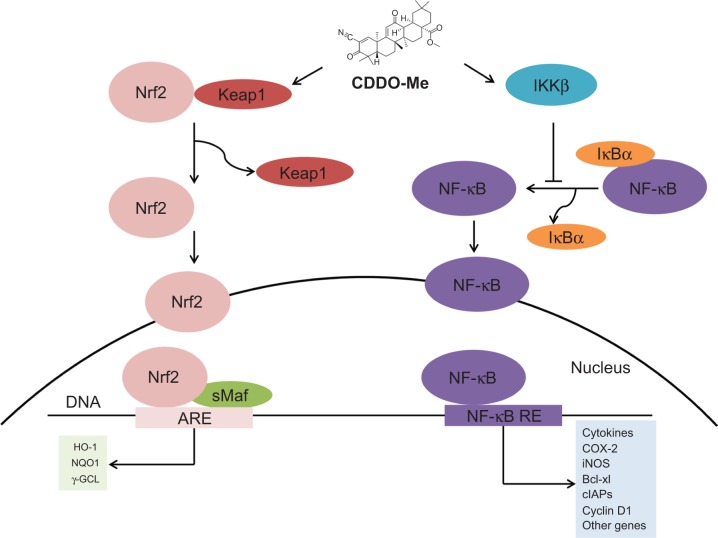
The main diseases targeted by Reata are all characterized by chronic inflammation and mitochondrial dysfunction, attributes that are addressed by activation of the NRF2 pathway (see figure below) [8]. In Alport syndrome, bardoxolone methyl is being studied in the pivotal Phase 2/3 CARDINAL trial to prevent the chronic inflammation that ultimately leads to progressive loss of kidney function [8]. The Phase 3 FALCON trial will test bardoxolone methyl for the treatment of ADPKD while the Phase 3 CATALYST trial will address the pathologic factors underlying CTD-PAH [8]. Omaveloxolone is undergoing testing in the Phase 2 MOXIe trial to target the chronic inflammation and mitochondrial dysfunction seen in Friedreich's ataxia with Reata recently announcing achievement of its primary endpoint of change in the modified Friedreich's Ataxia Rating Scale relative to placebo after 48 weeks of treatment [9].
Reata recently announced that they have reacquired the rights to bardoxolone methyl, omaveloxolone, and other next-generation Nrf2 activators from their previous collaborator AbbVie; certain Asian markets for bardoxolone are still licensed to their collaborator Kyowa Kirin Co., Ltd [10]. Upon release of this announcement, Reata stock increased 13% to $85.98 per a share, placing it among the biggest movers for shares of biotech companies; Chief Executive Warren Huff was quoted saying, "I think the deal and the positive reaction today reflect that confidence that these are very high-probability studies." [11]. Across all the potential uses Reata is studying, bardoxolone methyl could potentially treat up to 700,000 patients in the U.S. alone with the hope that approval of this drug could stave off dialysis for patients with kidney disease [11]. Chief Financial Officer Manmeet Soni estimated that these drugs could launch in late 2020 or early 2021, depending on the clinical results and Food and Drug Administration approval timelines [11].
- https://www.sec.gov/Archives/edgar/data/1358762/000156459019005161/reta-10k_20181231.htm
- https://www.eurekalert.org/pub_releases/2003-09/uots-ndb091603.php
- https://www.reatapharma.com/our-science/pipeline/
- https://www.reatapharma.com/our-science/our-technologies/
- https://www.ncbi.nlm.nih.gov/pubmed/28502971
- https://www.sciencedirect.com/science/article/pii/S2213231712000110#f0005
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4211867/
- https://www.reatapharma.com/diseases-we-target/disease-education/
- https://www.reatapharma.com/press-releases/reata-announces-positive-topline-results-from-the-moxie-registrational-trial-of-omaveloxolone-in-patients-with-friedreichs-ataxia/?releaseyear=2019
- https://www.reatapharma.com/press-releases/reata-pharmaceuticals-reacquires-rights-from-abbvie-to-develop-and-commercialize-bardoxolone-methyl-omaveloxolone-and-all-next-generation-nrf2-activators/?releaseyear=2019
- https://www.investors.com/news/technology/reata-stock-bullish-jump-biotech-company-relicenses-drugs-abbvie/#targetText=Under%20the%20terms%20of%20the,of%20%2475%20million%20in%202019.

